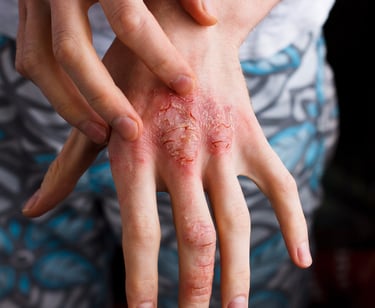
Moderate to Severe Atopic Dermatitis
Overview:
A Phase 2 Multicenter Platform Trial of Targeted Immunomodulator Therapies for Moderate to Severe Atopic Dermatitis
Inclusion Criteria:
Diagnosis of AD with onset of symptoms at least 1 year prior to the Baseline Visit and participant meets Hanifin and Rajka criteria.
Participant has applied non-medicated, additive-free bland emollient twice daily for at least 7 days before the Baseline Visit.
History of inadequate response to topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), or topical JAK inhibitors, OR systemic treatment for AD, OR participants for whom topical treatments are otherwise medically inadvisable (e.g., because of important side effects or safety risks).
Exclusion Criteria:
Use of the following AD treatments within the specified washout period prior to the Baseline Visit:
-- Systemic therapy for AD, including but not limited to corticosteroids, methotrexate, cyclosporine, azathioprine, phosphodiesterase type 4 (PDE4) inhibitors, IFN-γ, and mycophenolate mofetil within 5 half-lives [if known] or within 4 weeks, whichever is longer;
-- Any biologic treatments, (within 5 half-lives [if known]) or within 12 weeks (whichever is longer), or as specified below: < 8 weeks for dupilumab; < 12 weeks for nemolizumab; < 16 weeks for tralokinumab and lebrikizumab.
Phototherapy treatment, laser therapy, tanning booth, or extended sun exposure that could affect disease severity or interfere with disease assessments within 4 weeks.
Herbal treatments (e.g., traditional Chinese medicines) within 4 weeks.
Topical treatments (with the exception of non-medicated, additive-free bland emollients), including but not limited to TCS, TCIs, or topical PDE-4 inhibitors within 7 days.
Topical JAK inhibitor within 14 days.
Systemic JAK inhibitor (including but not limited to ruxolitinib, tofacitinib, baricitinib, upadacitinib, abrocitinib [PF-04965842], and filgotinib) within 5 half-lives [if known] or within 14 days, whichever is longer.
There are additional criteria that your study doctor will review with you to confirm you are eligible for the study.
Contact us to confirm eligibility
Contact Us
info@wscrinc.com
(303) 940-9773
Subscribe to our newsletter
We will not share your opt-in to an SMS campaign with any third party for purposes unrelated to providing you with the services of that campaign. We may share your Personal Data, including your SMS opt-in or consent status, with third parties that help us provide our messaging services, including but not limited to platform providers, phone companies, and any other vendors who assist us in the delivery of text messages. All the above categories exclude text messaging originator opt-in data and consent; this information will not be shared with any third parties.
